Letter from the President
 We hope all of our AAAB members have had a great start to the year so far!
We hope all of our AAAB members have had a great start to the year so far!
In this issue of AAAB Insights, check out the most recent regulatory updates out of Washington D.C. and across the country in Regulatory Updates and information from our most recent member committee meetings in Committee Updates. This month, we profile Cyber Security Solutions, one of our members in Member Highlight. Plan to attend one of our upcoming meetings after taking a look at the February calendar in the Members' Spot. If you would like to join a committee, please reach out and we can help you get involved.
For any questions or feedback regarding AAAB, please feel free to contact our leadership board via email at a.melgar@aaab.net.
Brandon Wood
AAAB President
Regulatory Updates
From DC:
Federal Regulations: In the month of January, there were 14 new Federal register entries in the Healthcare Reform section. Those entries break down as follows:
- Agency Information Collection Activities: Submission to OMB for Review and Approval; Public Comment Request; National Practitioner Data Bank: Modification of the National Practitioner Data Bank Code Lists (HRSA): HRSA, a sub-agency of HHS is announcing a change in the NPDB Basis for Action Code Lists for Federal Licensure, and State Licensure or Certification Actions to individuals and entities authorized to report and request information from the NPDB.
- Privacy Act of 1974; System of Records (SSA): In accordance with the Privacy Act of 1974, we are issuing public notice of our intent to modify an existing system of records entitled, Master Files of Social Security Number (SSN) Holders and SSN Applications (60-0058), last published on December 29, 2010.
- Agency Information Collection Activities: Proposed Collection; Comment Request (CMS): CMS is announcing an opportunity for the public to comment on CMS' intention to collect information from the public.
- Patient Protection and Affordable Care Act; HHS Notice of Benefit and Payment Parameters for 2023 (HHS): This proposed rule includes proposed payment parameters and provisions related to the risk adjustment and risk adjustment data validation programs, as well as proposed 2023 user fee rates for issuers offering qualified health plans through federally-facilitated Exchanges and State-based Exchanges on the Federal platform.
- Advisory Committee on Immunization Practices (CDC x 2): In accordance with the Federal Advisory Committee Act, the CDC announces the following meeting of the Advisory Committee on Immunization Practices.
- Agency Information Collection Activities: Proposed Collection; Comment Request (CMS): CMS is announcing an opportunity for the public to comment on CMS' intention to collect information from the public.
- Medicare Program; Contract Year 2023 Policy and Technical Changes to the Medicare Advantage and Medicare Prescription Drug Benefit Programs (CMS): This proposed rule would revise the Medicare Advantage (MA) (Part C) program and Medicare Prescription Drug Benefit (Part D) program regulations to implement changes related to marketing and communications, past performance, Star Ratings, network adequacy, medical loss ratio reporting, special requirements during disasters or public emergencies, and pharmacy price concessions.
- Updates to the Bright Futures Periodicity Schedule (HRSA): Effective December 30, 2021, HRSA accepted recommended updates to the Bright Futures Periodicity Schedule, a HRSA-supported guideline for infants, children and adolescents for purposes of ensuring that non-grandfathered group and individual health insurance issuers provide coverage without cost sharing under the Public Health Service Act.
- Medicare Program; Updates to Lists Related to Durable Medical Equipment, Prosthetics, Orthotics, and Supplies (DMEPOS) Conditions of Payment (CMS): This document announces the updated Healthcare Common Procedure Coding System (HCPCS) codes on the Master List of DMEPOS Items Potentially Subject to Face-to-Face Encounter and Written Order Prior to Delivery and/or Prior Authorization Requirements.
- Announcement of the Advisory Panel on Outreach and Education (APOE) February 3, 2022 Virtual Meeting (CMS): This notice announces the next meeting of the APOE (the Panel) in accordance with the Federal Advisory Committee Act.
- Agency Information Collection Activities: Proposed Collection; Comment Request (CMS): CMS is announcing an opportunity for the public to comment on CMS' intention to collect information from the public.
- Coronavirus State and Local Fiscal Recovery Funds (Treasury): This rule implements the Coronavirus State Fiscal Recovery Fund and the Coronavirus Local Fiscal Recovery Fund established under the American Rescue Plan Act.
- Introduction to the Unified Agenda of Federal Regulatory and Deregulatory Actions-Fall 2021 (RISC): The Regulatory Flexibility Act requires that agencies publish semiannual regulatory agendas in the Federal Register describing regulatory actions they are developing that may have a significant economic impact on a substantial number of small entities (5 U.S.C. 602).

ACA:
- Medicaid Expansion: In Wyoming, the Joint Revenue Interim Committee has sponsored a Medicaid expansion bill that will be implemented if the condition of the federal government continuing to pay 90 percent of the cost is met. In Missouri, it has been reported that Medicaid Expansion enrollment is not as high as expected. After a Missouri judge ruled that the state must begin enrolling residents, enrollments only went up by 20 percent. Out of the 274,000 eligible to sign up for Medicaid in Missouri since expansion in October 2021, only 53,000 have enrolled.
- Open Enrollment Final Numbers: The Biden Administration announced that a record-breaking 14.5 million people have signed up for 2022 health care coverage through HealthCare.gov during the Open Enrollment Period (OEP) from November 1, 2021 through January 15, 2022 – including 5.8 million people who have newly gained coverage during 2021. HealthCare.gov consumers saw their average monthly premium fall by 23%, compared to the previous year.
Medicare:
- Value-based Care: The Center for Medicare and Medicaid Innovation (CMMI) wants to replace or improve its risk adjustment methodology after concerns of Medicare Advantage plans upcoding to get higher payments, the center’s director said. CMMI Director Liz Fowler, Ph.D., detailed how the center plans to shift the focus in its payment models to care delivery during a webinar sponsored by the advocacy group United States of Care. The remarks come as scrutiny over MA coding practices has loomed over the lucrative market.
- Medicare Advantage Complaints Up: CMS recently released a proposed rule that disclosed that complaints are up over 100% year over year. In 2020, CMS received a total of 15,497 complaints related to marketing but in 2021, excluding December, the total was 39,617.
Around the Country:
- No Surprise Act (NSA) Implemented: Balance billing is now prohibited for out of network charges submitted by a provider at a qualifying in network facility; and for essentially all emergency services, though some exceptions still exist (like ground ambulance). The NSA has broad payor coverage -- all the way from small to large group health plans, self-insured markets, and plans grandfathered in under the ACA. But the NSA does not apply to FFS Medicare, Medicare Advantage or Managed Medicaid plans. The NSA requires hospitals, physicians and insurers to agree on these ‘in-network rates’ while holding the patient harmless. Out-of-network doctors must inform their patients about what their care might cost and may ask patients to sign a form that waives their protections. Lastly, patients can direct complaints online or through a 1-800 number.
- Big Pharma Win: The Biden Administration decided to pull the Trump Administration’s proposed rule that tied U.S. drug pricing to rates paid in other countries. Basically, the American consumer has been subsidizing world-wide drug prices for years and this rule would’ve moderated that. The reason cited was that the rule spurred the filing of four lawsuits from the pharmaceutical industry, which resulted in a legal stay delaying it from going into effect.
- Cyber Breech: Hackers breached the computer networks of Broward Health in October and may have accessed personal and financial information on more than 1.3 million patients and staff. The southeast Florida health system, which operates more than 30 healthcare locations in Broward County, Florida disclosed it was hit with a cyberattack on Oct. 15, 2021, when an intruder gained unauthorized access to the hospital's network and patient data through a third-party medical provider, according to a statement posted to the health system's website. The health system said it discovered the intrusion four days later, on Oct. 19, and contained the incident, then notified the FBI and the Department of Justice (DOJ). Broward Health said it waited months to notify victims and make the breach public because the DOJ told them to hold off on sending out breach notification letters to preserve an ongoing law enforcement investigation.
- 2021 Milliman Medical Index (MMI) Released: In 2020 according to the MMI, healthcare costs decreased year over year 4.2% from 2019 for the first time in the history of the MMI. This drop was accounted for by the elimination and deferral of significant amounts of care that more than offset the cost of COVID-19 testing and treatments. However, this went the opposite direction for 2021: The MMI projects healthcare costs have grown approximately 8.4% from 2020 to 2021. This increase was driven by a rebound in healthcare utilization which is higher than historical healthcare cost increases and GDP growth over the past five years.
- Almost 13 Million Americans Skipped Prescription Drugs Due to Costs Before COVID: About 12.8 million adults delayed or did not get prescription drugs in 2018-19 due to costs, including about 3.8 million privately insured nonelderly adults and 2.3 million elderly Medicare beneficiaries, according to a study by the Robert Wood Johnson Foundation and Urban Institute based on 2018–19 data from the Medical Expenditure Panel Survey. About 9.5% of adults who were uninsured all year reported unmet prescription drug needs, compared with 4.9% of Medicare beneficiaries and 5.6% of nonelderly Medicaid enrollees. More than one-quarter of adults with Medicare and 5.3% of privately insured people spent more than 1% of their family incomes on out-of-pocket prescription drug costs.
- Acquisition: Vera Whole Health, the advanced primary care provider, has announced that it plans to acquire healthcare data platform, Castlight Health, for $370 million. The two organizations stated their belief that the acquisition will facilitate more “personalized” and better coordinated care for patients. The combined company will be held privately, removing shares of Castlight which are currently traded publicly. Vera’s majority owners have also pledged to invest up to $338 million in the new combined entity. The announcement also included news of investment support from Anthem, one of Castlight’s largest customers.
- Drug Overcharges: Centene reached its latest settlement over alleged PBM overcharging and agreed to pay New Hampshire $21M. The insurer has now settled with at least five states, paying out more than $236 million. It's possible more settlements are on the horizon, as Centene has set aside $1.1 billion for future payments.
- Shkreil Fine: AP reported that Martin Shkreli must return $64.6 million in profits he and his former company reaped from jacking up the price and monopolizing the market for a lifesaving drug, a federal judge ruled while also barring the provocative, imprisoned ex-CEO from the pharmaceutical industry for the rest of his life.
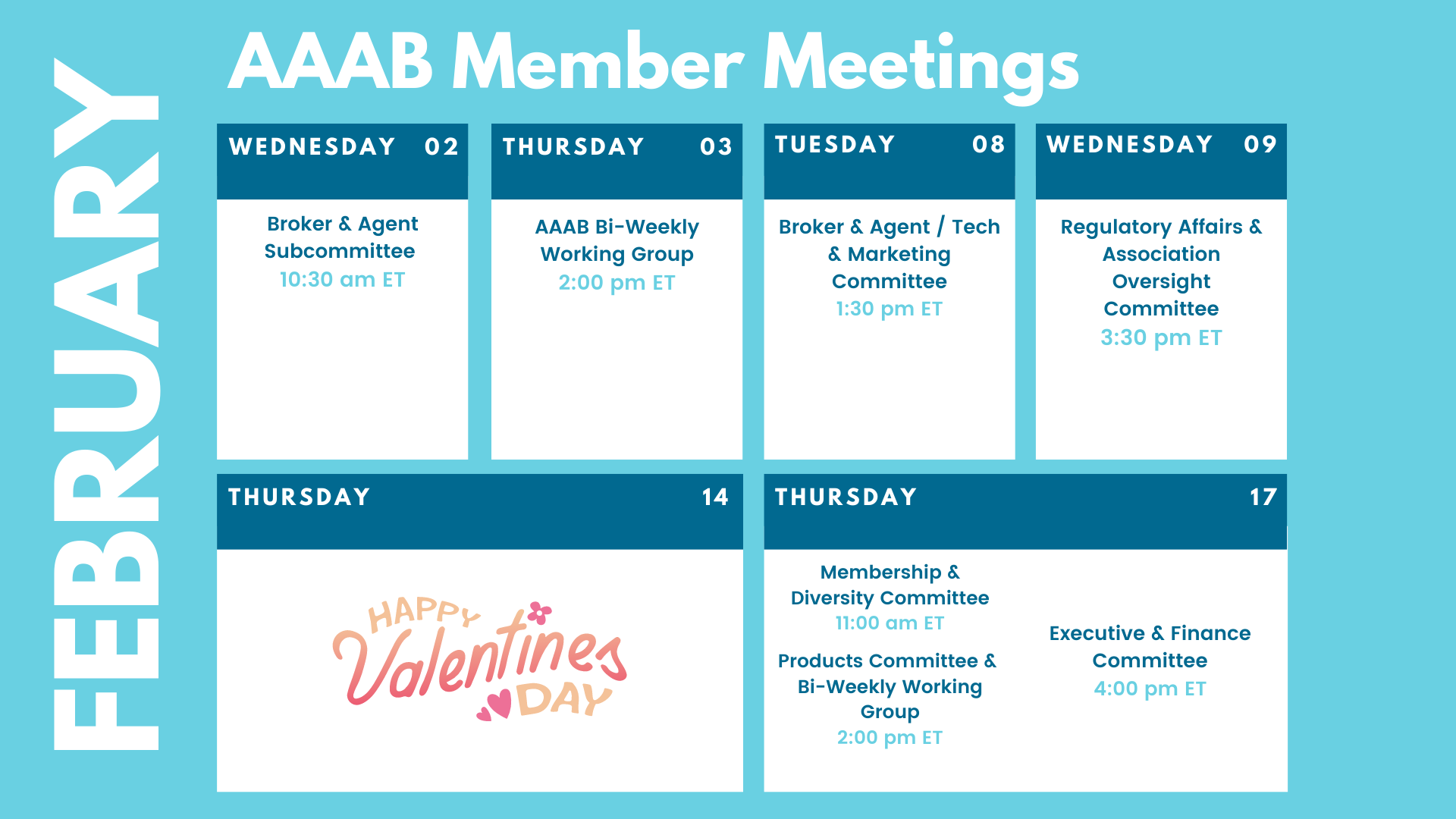
Committee Updates
Committee Updates: :
- Broker Committee: Discussed building out a compliance toolbox that will encompass the TCPA toolbox that was originally worked.
- Independent Agent Subcommittee: Member Dee Hohenberger has arranged a deal with NAPA that the board has approved so AAAB can offer its E&O coverage to its future independent agent members.
- Membership/Diversity Committee: Continues to build a pipeline of prospects and work those prospects to join. Christa Rappaport has agreed to provide leadership to our diversity subcommittee of the membership committee.
- Product Committee: John Francini is working with NAIC contacts to obtain product data that the association members can use.
- Regulatory Affairs Committee: Reviewed federal notices and decided to respond to the proposed rule on Medicare Advantage marketing.

Member Highlight
In order for all of us to get to know our diverse member base, we are highlighting a member each month. This month we want to introduce Cyber Security Solutions.
![]() Cyber Security Solutions believes in creating new technologies by enabling the innovative minds that make it happen. While many companies focus on profit and loss statements, CSS focuses on your security. It is their mission to protect your data, and it is their goal to make sure it is affordable enough to implement for any organization. CSS strives on finding ways to ensure that any small-medium business can afford to protect their data and the data of their customers. CSS develops their own solutions that includes all the security tools your organization needs so that you can focus on one vendor for your entire security program.
Cyber Security Solutions believes in creating new technologies by enabling the innovative minds that make it happen. While many companies focus on profit and loss statements, CSS focuses on your security. It is their mission to protect your data, and it is their goal to make sure it is affordable enough to implement for any organization. CSS strives on finding ways to ensure that any small-medium business can afford to protect their data and the data of their customers. CSS develops their own solutions that includes all the security tools your organization needs so that you can focus on one vendor for your entire security program.
At CSS, their experts know just what to do about your alphabet soup of regulations. CSS provides you with a solution that ensures you obtain and maintain a secure and compliant environment. Security is key, and if you are secure you will be compliant with any regulation. They are not handing you a cookie-cutter solution. Your plan with CSS will be tailored to your business, budget, and compliance requirements. You have important work to do—CSS will take care of the rest.
Members' Spot
Upcoming Member Opportunities
Get involved! If you aren't currently serving on a committee, but would like to, please email a.melgar@aaab.net.